Did you know Trodelvy (sacituzumab govitecan) remains detectable in the bloodstream for up to 18 days after infusion? This targeted therapy, approved for metastatic triple-negative breast cancer, works differently than traditional chemotherapy. Knowing its duration in the body helps patients and doctors manage treatment schedules and potential side effects.
The medication is administered intravenously in outpatient clinics, typically over 3 hours on days 1 and 8 of a 21-day cycle. Initial infusions may take longer to monitor for reactions. Pharmacokinetics – how the drug moves through the system – depend on liver function, body weight, and other health factors.
This guide explains key details about Trodelvy’s treatment timeline. You’ll learn about metabolism patterns, common monitoring practices, and why clearance rates matter for dosing adjustments. We’ll also explore how healthcare teams personalize care based on individual patient needs.
Key Takeaways
- Trodelvy’s scientific name is sacituzumab govitecan
- Used primarily for metastatic breast and bladder cancers
- Administered through IV infusion in clinical settings
- Body clearance time varies between patients
- Regular blood tests help track medication levels
- Treatment cycles repeat every 21 days
Introduction to Trodelvy and Its Role in Breast Cancer Treatment
Trodelvy represents a breakthrough in precision medicine for challenging breast cancer subtypes. Approved by the FDA in 2020, this antibody-drug conjugate specifically targets triple-negative breast cancer (TNBC) and HR-positive, HER2-negative metastatic breast cancers. These aggressive forms often resist standard therapies, making Trodelvy a vital option for patients with limited alternatives.
Administered through outpatient IV infusions every three weeks, treatment involves careful coordination. Nurses monitor patients during the 3-hour sessions while pharmacists prepare doses based on body weight. “Regular blood work helps us adjust treatment plans proactively,” explains Dr. Lisa Hamilton, an oncology specialist at Memorial Sloan Kettering.
Key aspects of Trodelvy therapy include:
- Targeted action against TROP-2 proteins abundant in cancer cells
- Combined approach: antibody-guided delivery + tumor-killing payload
- Multidisciplinary care teams managing side effects and progress
Healthcare providers use imaging scans and symptom tracking alongside lab results to evaluate effectiveness. This comprehensive strategy allows personalized adjustments while maintaining treatment continuity.
What is Sacituzumab Govitecan? Understanding Trodelvy
Sacituzumab govitecan serves as the scientific name for Trodelvy, a dual-action cancer treatment merging precision targeting with chemotherapy. This antibody-drug conjugate specifically tackles aggressive cancers that resist standard therapies. Its design focuses on minimizing harm to healthy cells while maximizing tumor destruction.
The drug contains two key components. A monoclonal antibody binds to Trop-2 proteins found abundantly on cancer cells. Attached to this antibody is SN-38, a potent chemotherapy agent derived from irinotecan. This combination allows precise delivery of cancer-killing compounds directly to tumors.
| Component | Function | Target |
|---|---|---|
| Sacituzumab (antibody) | Identifies cancer markers | Trop-2 proteins |
| Govitecan (SN-38) | Destroys tumor cells | DNA replication process |
Clinical use focuses on metastatic or surgically inoperable cancers, particularly those lacking treatment options. The therapy shows greatest effectiveness in cancers with high Trop-2 expression, including certain breast and bladder variants.
Unlike traditional chemotherapy that affects all rapidly dividing cells, this targeted approach reduces collateral damage. Ongoing research continues to explore applications for other Trop-2 rich malignancies, potentially expanding its therapeutic reach.
how long does trodelvy stay in your system
Patients beginning this therapy often ask about the schedule and duration of medication effects. The first dose typically requires three hours for administration, with follow-up sessions sometimes reduced to one or two hours. This adjustment occurs once healthcare teams confirm tolerability.
Treatment follows a structured 21-day cycle. Initial doses occur on day 1, with a second infusion seven days later. The remaining two weeks allow recovery before restarting the process. Care teams monitor blood counts during this period to assess medication clearance.
Three key factors influence treatment planning:
- Individual metabolism rates affecting drug processing
- Body composition impacting medication distribution
- Organ function (particularly liver health) guiding clearance speed
Understanding these timelines helps patients prepare for appointments and manage side effects. Those with slower clearance may experience prolonged medication activity, requiring closer monitoring. Treatment FAQs provide additional guidance for scheduling concerns.
Regular blood tests track how quickly the therapy leaves the bloodstream. This data helps oncologists optimize dosing intervals while maintaining effectiveness. Most patients complete multiple cycles unless significant side effects develop.
Pharmacokinetics and Dosing Cycle Insights
Understanding Trodelvy’s journey through the body helps optimize its cancer-fighting potential. Pharmacokinetics reveal how the medication absorbs into tissues, distributes to tumor sites, and clears through liver pathways. These processes determine both effectiveness and safety during treatment cycles.
Infusion Duration and Cycle Structure
Patients receive chemotherapy infusions on days 1 and 8 of a 21-day treatment cycle. Initial sessions last three hours to monitor for allergic reactions. Subsequent doses often shorten to 1-2 hours once tolerance is confirmed.
Care teams adjust schedules based on blood counts and side effects. “We prioritize safety without compromising efficacy,” notes Dr. Emily Carter, an oncology pharmacist. Fever or severe fatigue may prompt temporary dose reductions until symptoms improve.
Factors Influencing Drug Clearance
Three elements shape how quickly the body processes Trodelvy:
- Liver function: Primary clearance pathway
- Body mass: Impacts medication distribution
- Concurrent treatments: Potential metabolic interactions
Regular blood tests track drug levels between cycles. Patients with slower clearance may require extended recovery periods. This personalized approach maintains chemotherapy benefits while minimizing risks.
Administration Methods and Treatment Settings
Proper medication delivery plays a critical role in cancer treatment effectiveness. Trodelvy requires specialized intravenous methods to ensure precise dosing and patient safety. Healthcare teams tailor administration based on individual needs and treatment duration.
Outpatient Infusion Process
Patients receive treatment in clinic-based day units equipped for chemotherapy administration. Before each session, nurses check vital signs and review recent blood work. Pharmacists prepare doses using strict safety protocols to maintain drug potency.
The care team monitors patients throughout the 1-3 hour infusion. Immediate access to emergency equipment ensures rapid response to rare allergic reactions. Most individuals return home the same day after post-infusion observation.
Intravenous Delivery Techniques
Four primary IV access methods support Trodelvy administration:
- Peripheral cannula: Short-term needle for single sessions
- PICC line: Semi-permanent arm catheter for multiple cycles
- Portacath: Surgically implanted chest device for long-term use
- Central line: Neck or groin access for urgent needs
Oncologists select the best option based on vein health and treatment duration. Proper technique prevents medication leakage and protects surrounding tissue. Regular line maintenance by nurses reduces infection risks during extended therapy.
Mechanism of Action: Targeting TROP2 for Cancer Therapy
Trodelvy’s cancer-fighting strategy centers on a protein called TROP2, commonly found in large amounts on aggressive tumor cells. This target-seeking approach allows precise delivery of chemotherapy while sparing healthy tissues—a major advancement for hard-to-treat cancers.
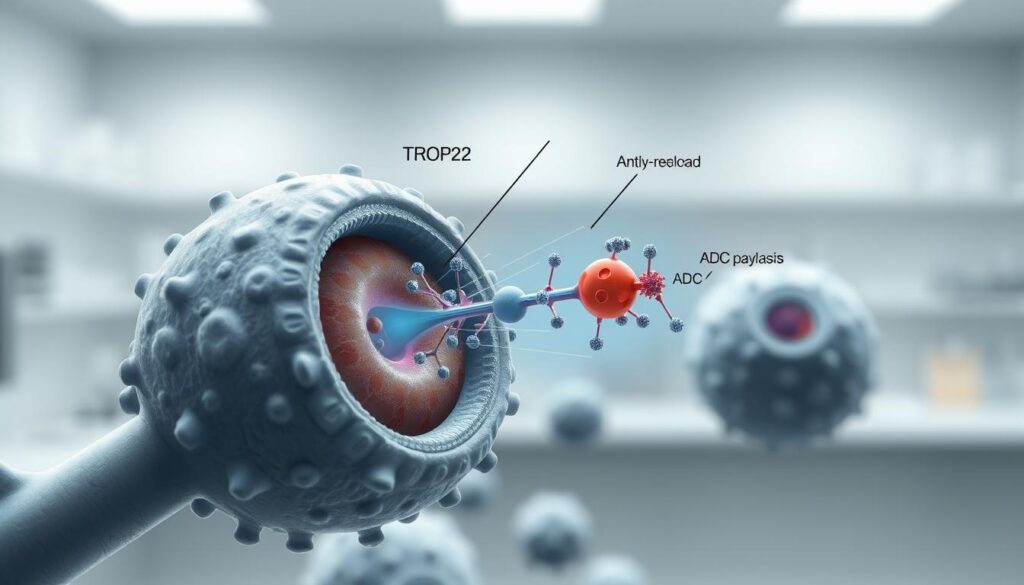
The treatment works through a two-part process. First, its antibody component locks onto TROP2 proteins like a key fitting a lock. Once attached, the cancer cell absorbs the entire compound, releasing the potent SN-38 chemotherapy agent directly into the tumor.
Three key features make this mechanism effective:
- High-affinity binding to TROP2 receptors
- Controlled release of SN-38 inside cancer cells
- Bystander effect reaching neighboring cells
The bystander effect proves particularly valuable. As SN-38 leaks from destroyed cancer cells, it penetrates nearby tumors regardless of their TROP2 levels. This expands treatment impact beyond directly targeted areas.
For triple-negative breast cancer (TNBC) patients, this approach addresses a critical need. TNBC tumors often lack the hormone receptors targeted by other therapies, making TROP2 an ideal focus. Clinical trials show improved outcomes when using this protein-specific strategy compared to traditional chemotherapy.
Unlike conventional treatments that affect all rapidly dividing cells, this targeted action reduces damage to healthy tissues. This precision translates to fewer side effects while maintaining strong anti-cancer activity—a balance crucial for patients undergoing extended treatment cycles.
Treatment Scheduling and Timing Considerations
Structured treatment plans help balance cancer-fighting power with patient safety. The standard regimen follows 21-day cycles, with infusions typically administered on days 1 and 8. This schedule allows medication activity peaks while providing recovery time between doses.
Cycle Intervals and Dose Timing
Each cycle begins with careful blood work to assess medication levels and organ function. Nurses administer infusions through IV ports, monitoring for immediate reactions. Patients typically continue this pattern for several months, provided scans show tumor response and side effects remain manageable.
Three factors determine schedule adherence:
- White blood cell counts between cycles
- Liver enzyme levels affecting drug processing
- Patient-reported symptoms like fatigue or nausea
Adjustments Based on Treatment Response
Oncologists modify schedules when early signs of adverse effects appear. Dose delays of 1-2 weeks help patients recover from low platelet counts or diarrhea. Treatment may pause entirely if symptoms persist beyond manageable levels.
Regular imaging scans guide long-term planning. Some patients maintain the regimen for 6-12 months if tumors keep shrinking. “We prioritize sustained quality of life while pushing for maximum effectiveness,” explains Dr. Rachel Nguyen, a breast cancer specialist at MD Anderson.
Monitoring Treatment Progress and Response
Effective cancer care requires vigilant tracking of both treatment impact and patient health. Care teams use a dual approach—lab tests and imaging—to assess how well therapy works while safeguarding wellness. This strategy helps balance aggressive cancer control with quality-of-life preservation.
Regular Blood Test Monitoring
Blood work forms the frontline defense against treatment complications. Weekly checks track white blood cell counts, liver enzymes, and kidney function. “Our team prioritizes early detection of complications through scheduled check-ins,” notes Dr. Sarah Mitchell, an oncologist at Dana-Farber Cancer Institute.
Neutropenia prevention remains critical. Low white blood cell levels increase infection risks, potentially delaying treatment. Care teams may prescribe growth factor injections or adjust doses when counts drop below safe thresholds.
Imaging and Clinical Assessments
CT scans and MRIs provide visual proof of tumor changes every 2-3 months. Radiologists compare images to measure shrinkage or stability. Meanwhile, nurses document physical symptoms like fatigue patterns or pain levels during clinic visits.
Three key indicators guide adjustments:
- Persistent blood count abnormalities
- Scan results showing limited tumor response
- Patient-reported side effects affecting daily life
This collaborative approach lets teams modify plans before minor issues become major setbacks. Patients play a vital role by promptly reporting new symptoms between appointments.
Managing Common Side Effects and Patient Wellbeing
Patients undergoing cancer treatment often face temporary but challenging physical reactions. Managing these effects improves treatment continuity and quality of life. Healthcare teams use proactive strategies to address expected symptoms while preventing complications.

Neutropenia and Infection Risk
Low white blood cell counts occur in 60% of patients, increasing infection risks. Doctors prescribe growth factor injections to boost immune cell production. Regular blood tests help detect neutropenia early.
| Side Effect | Management Strategy | When to Contact Team |
|---|---|---|
| Fever over 100.4°F | Immediate antibiotics | Within 1 hour |
| Mouth sores | Saltwater rinses | Persisting 3+ days |
| Fatigue | Activity pacing | Preventing daily tasks |
Nausea, Diarrhea, and Other Reactions
Anti-nausea medications given before infusions prevent stomach upset in 75% of cases. For diarrhea, hydration solutions and dietary changes often help. Hair thinning typically begins 2-3 weeks after starting treatment.
Severe allergic reactions during IV sessions remain rare but require immediate attention. Clinics keep epinephrine ready for rapid response. Patients should report rashes or breathing changes immediately.
Open communication enables timely adjustments. Dose reductions or schedule changes help 40% of patients manage persistent symptoms. Care teams prioritize maintaining treatment effectiveness while minimizing discomfort.
Integrating Supportive Care and Symptom Relief Strategies
Comprehensive cancer care extends beyond medication to address treatment-related challenges. Combining symptom management with therapeutic interventions helps patients maintain treatment continuity while preserving quality of life. Supportive strategies work alongside cancer therapy to reduce discomfort and enhance daily functioning.
Common side effects like nausea and diarrhea often respond well to targeted interventions. Anti-diarrheal medications and IV hydration help manage gastrointestinal issues. Nutritional counseling may also improve energy levels through balanced meal planning tailored to treatment phases.
| Side Effect | Management Approach | Additional Support |
|---|---|---|
| Fatigue | Scheduled rest periods | Gentle stretching routines |
| Mouth sores | Medicated rinses | Soft-food diets |
| Skin reactions | Topical creams | Cool compresses |
Healthcare teams create personalized plans addressing individual needs. Some patients benefit from acupuncture for neuropathy, while others prioritize sleep hygiene adjustments. Regular check-ins allow quick strategy modifications as treatment progresses.
Lifestyle changes may also complement medical care. Staying hydrated supports kidney function during therapy. Small, frequent meals often work better than traditional portions. Open communication with care providers ensures strategies evolve with changing needs.
These integrated approaches help patients navigate treatment while managing physical demands. Collaborative planning between medical teams and individuals fosters resilience during cancer care journeys.
Lifestyle Considerations During Trodelvy Treatment
Daily choices significantly influence how patients experience cancer therapy. Simple adjustments to nutrition, activity levels, and self-care routines can enhance treatment tolerance and recovery. These strategies become especially important for individuals managing breast cancer therapies that impact multiple body systems.
Fueling the Body Strategically
Balanced meals help combat common effects like nausea and fatigue. Consider these approaches:
- Small, frequent meals instead of large portions
- High-protein snacks between infusions
- Hydration tracking using marked water bottles
Some patients find ginger tea eases stomach discomfort. Avoid spicy or greasy foods if diarrhea occurs. Dietitians often recommend potassium-rich bananas and bland carbohydrates during challenging days.
Movement and Recovery Balance
Light exercise maintains strength without overtaxing the body. Try:
- 10-minute walks after morning rest
- Chair yoga for joint flexibility
- Breathing exercises to reduce stress
Track energy patterns using a journal. Schedule demanding tasks for high-energy periods. Most patients need 8-10 hours of sleep nightly, with daytime naps as needed.
Gentle skin care prevents irritation from therapy-related dryness. Use fragrance-free cleansers and pat skin dry instead of rubbing. Moisturize immediately after bathing while pores remain open. Sun protection becomes crucial—apply SPF 30+ even on cloudy days.
Safety Information and Essential Reminders for Patients
Vigilance during cancer treatment protects both treatment outcomes and patient health. Care teams prioritize early detection of adverse reactions through structured protocols and patient education. Awareness of potential risks allows timely intervention when challenges arise.
Preventing Allergic and Infusion Reactions
Common side effects like mild itching or flushing may occur during IV sessions. Severe reactions involving throat swelling or breathing difficulties require immediate action. Clinics pre-medicate patients with antihistamines to reduce risks.
Critical precautions include:
- Reporting prior allergies to medications or contrast dyes
- Staying hydrated before infusions to support vein access
- Wearing loose clothing for easy monitoring access
Patients with triple-negative breast cancer or those who’ve had surgery removed lymph nodes need special monitoring. Swelling at the infusion site could indicate vein irritation. Care teams keep emergency medications on hand for rapid response.
| Reaction Type | Warning Signs | Action Required |
|---|---|---|
| Mild | Localized rash | Antihistamine dose |
| Severe | Wheezing, dizziness | Stop infusion + epinephrine |
Contact your oncology team immediately if you develop fever above 100.4°F or sudden fatigue. Those with surgery removed drainage nodes should watch for arm swelling. Regular blood tests between cycles help track treatment safety for negative breast cancer patients.
Conclusion
Navigating cancer treatment requires balancing scientific precision with personalized care. This therapy’s unique design—targeting specific proteins while sparing healthy cells—explains its role in managing aggressive cancers. Treatment timing and clearance patterns directly impact safety, making regular blood tests and imaging scans essential.
Proper administration through IV infusions ensures optimal drug delivery. Care teams monitor for swelling at injection sites and manage common reactions like skin rash through proactive strategies. Supportive care measures, including dietary adjustments and symptom tracking, help maintain treatment continuity.
Patients should promptly report unexpected rash development or persistent swelling to their oncology team. Open communication enables timely dose adjustments and side effect management. Combined with lifestyle considerations, these practices help optimize outcomes while preserving quality of life.
Always consult your healthcare providers about medication timelines and skin rash prevention techniques. Their expertise guides safe, effective treatment journeys tailored to individual needs.

